January 20, 2021 | 16:07 (GMT+7)
Human trials of third homegrown COVID-19 vaccine to begin in March
A third COVID-19 vaccine developed by Vietnam is scheduled to be tested on humans at the end of March, the Ministry of Health said on January 19.
The vaccine is developed by Vaccine and Biological Production No. 1 (VABIOTECH). It is one of the four companies that are developing COVID-19 vaccines in Vietnam, along with the Institute of Vaccines and Medical Biologicals (IVAC), the Centre for Research and Production of Vaccines and Biologicals (POLYVAC), and Nanogen Pharmaceutical Biotechnology (NANOGEN).
    |
 |
|
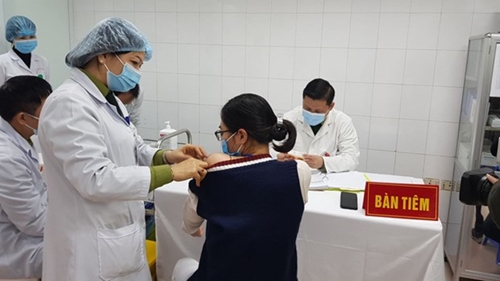
The highest dose, 75mcg, of Nanocovax was given to three volunteers in Hanoi on January 12. |
Nanocovax by NANOGEN is Vietnam’s first candidate vaccine against the novel coronavirus SARS-CoV-2 to reach the human trial stage.
The highest dose, 75mcg, of Nanocovax was given to three volunteers in Hanoi on January 12.
The Nha Trang-based IVAC has also been authorized to carry out human clinical tests for its Covivac.
According to Associate Professor Dr Chu Van Men, Director of the Military Medical University’s Centre for Clinical Trials and Bioequivalence, Vietnam expects to collect all necessary data on clinical testing by the end of 2021 before it can consider mass vaccination.
Source: VNA