The three women, aged 20 to 22, are the first of a total of 20 volunteers in the testing program.
Following their injection, the volunteers will undergo health monitoring for 72 hours at the Hanoi-based Military Medical University. The other 17 volunteers will receive injections if the vaccine’s safety is confirmed.
    |
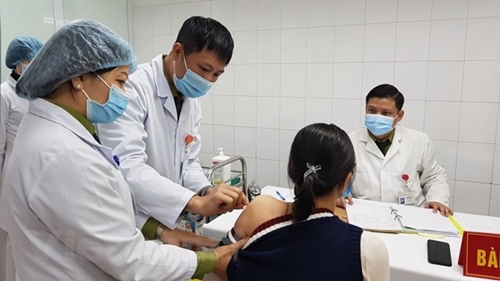 |
|
A volunteer is given a shot of the 75mcg dose of Nanocovax on January 12. |
Two groups of 20 people each are testing the 25mcg and 50mcg doses, receiving their first injections on December 17 and 26, respectively.
Pain at the injection site and a light fever lasting 24 hours were the only two issues reported by volunteers, according to Associate Prof., Dr. Chu Van Men, Director of the Military Medical University’s Center for Clinical Trials and Bioequivalence. All are now in a stable condition, he added.
The volunteers testing the 25mcg doses are scheduled to take their second shot on January 15 or 16.
According to Men, this first clinical trial, which tests the vaccine’s safety and identifies the optimal dosage, is 50 percent complete.
Vietnam expects to collect all necessary data on clinical testing by the end of 2021 before it can consider mass vaccination, he said.
Developed by the Nanogen Pharmaceutical Biotechnology JSC and the Vietnam Military Medical University, Nanocovax is Vietnam’s first candidate vaccine against the novel coronavirus to reach the human trial stage.
Vietnam has several other COVID-19 candidate vaccines being developed, by Vabiotech, Polyvac, and the Institute of Vaccines and Medical Biologicals.
Source: VNA