Developed by the Nanogen Pharmaceutical Biotechnology JSC and the Vietnam Military Medical University, Nanocovax is Vietnam's first COVID-19 candidate vaccine to reach the human trial stage.
A total of 60 volunteers, aged 18-50, were selected for the first phase of the clinical trials. They are divided into three groups for receiving three doses of 25 mcg, 50 mcg and 75 mcg, respectively. The vaccination consists of two injections 28 days apart.
    |
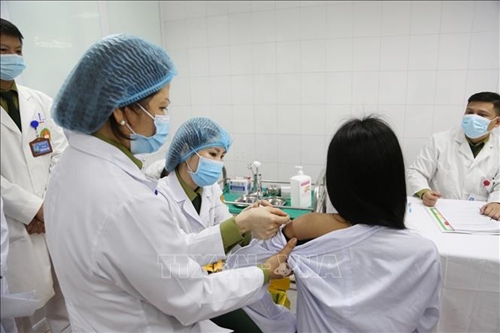 |
|
A female volunteer receives the first shot of a dose of 50mcg of Nanocovax COVID-19 vaccine |
After the injection, the volunteers have been put under health monitoring at the Hanoi-based Military Medical University for 72 hours. The remaining 17 in the group will get injections of the 50mcg dose if the vaccine’s safety is confirmed.
The first group testing the 25 mcg dose received the first injections on December 17. According to Major General Hoang Van Luong, Deputy Director of the university, after the shots, all the 20 volunteers in the group are in stable condition with no abnormal signs detected.
The first phase is scheduled to end after a month and a half, during which the university will continue to select suitable volunteers for the second, which is scheduled to begin in March 2021.
The participants will be under medical monitoring for six months from the day they were given the first injection.
The Nanogen Pharmaceutical Biotechnology JSC has spent 20 billion VND (865,946 USD) buying a health insurance package for the volunteers.
Apart from Nanocovax, Vietnam has several other COVID-19 candidate vaccines being developed by Vabiotech, Polyvac, and the Institute of Vaccines and Medical Biologicals.
Source: VNA