Nano Covax is the first Vietnam-made COVID-19 vaccine and is manufactured by Nonogen Biotechnological Joint-Stock Company.
Absolute safety for volunteers is first and foremost priority
Doctor Nguyen Ngo Quang, Deputy Director of the Science, Technology and Training Department of the Health Ministry released that the Military Medical Academy conducted the first phase trial of Nano Covax on humans on the morning of December 17 at the Military Medical Academy. The three volunteers, including two men and one woman received the first injections of the Vietnam-made COVID-19 vaccine.
After receiving the injections, the volunteers have been carefully examined and cared for by doctors at the Military Medical Academy. The trial was witnessed by health officials and experts. Results of the post-injection examination showed that the health of all three volunteers is good.
    |
 |
|
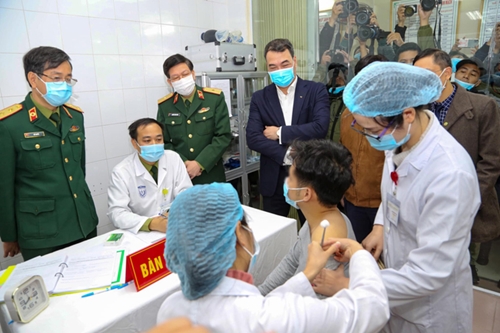
The first three Vietnamese people to receive injections of Nano Covax (Vietnam-made COVID-19 vaccine) received the injections on December 17 |
Doctor Nguyen Ngo Quang also stressed that the entire process of the first phase Nano Covax human trial will be strictly supervised by three teams of independent medical experts to ensure that the results of the trial will be objective, realistic and reliable.
Regarding the procedures for the first trial of Nano Covax on humans, the health official said that the Military Medical Academy is a leading medical center in the country, with high-profile researchers, competent medical experts and experienced practitioners so it has been selected to conduct the vaccine trial. So far, military doctors have strictly implemented all medical procedures.
Regarding the risk of the volunteers’ health, Doctor Nguyen Ngo Quang said that the Ministry of Health, the Military Medical Academy, the Nano Covax producer and participating doctors have given the first and foremost priority to ensuring the absolute safety and health of the volunteers for the vaccine testing. “In the first phase, we attach much importance to ensuring the safety and health of the volunteers. The efficiency and immunity of the vaccine will be taken into account in the second and third phases of the Nano Covax human trial,” Doctor Nguyen Ngo Quang explained
According to him, the three volunteers are now cared for by doctors of the Medical Military Academy, and the intensive care at the hospital will last 72 hours after the injections. After that, the volunteers will go home, and they will be supervised and cared for by local medical workers.
Health of Nano Covax tested volunteers carefully cared by doctors
Rector of the Military medical Academy, Lieutenant General Do Quyet, Dr., Prof. said that the academy and the Nanogen Company have made careful preparations for the clinical trials.
“The academy has earmarked the best equipment and personnel for the clinical trials. The volunteers are monitored by medical doctors and experts around the clock at Military hospital 103. Doctors frequently examine their health during the first 72 hours after the injections, ensuring that all of their health changes and reactions will be recorded and handled in an immediate and effective manner. We have also reserved an emergency room with a team of competent medical workers to deal with any unexpected situations,” Rector of the Military Medical Academy Do Quyet said.
Assessing the vaccine of Nano Covax, he said, the Vietnam-made COVID-19 vaccine has been rated as safe for humans.
“If the three volunteers do not have major side effects and their health is good, they will receive the second injection of Nano Covax after 28 days,” the Rector of the Military Medical Academy said.
According to a leader of Military Hospital 103, some 300 people have volunteered themselves for the first phase of the clinical trial of Nano Covax.
The first phase of the clinical trial runs from December 2020 to February 2021 in Hanoi while the second phase will be conducted from February 2021 to August 2021, and the third phase from August 2021 to February 2022. The first phase needs some 300 volunteers while the second phase and third phase respectively need 400-600 volunteers and 1,500-3,000 volunteers.
Translated by Thu Nguyen