Nanocovax, developed by the Nanogen Pharmaceutical Biotechnology JSC, is the first Vietnam-made COVID-19 vaccine to enter human trials, with another two from other manufacturers to follow in February and March 2021.
    |
 |
|
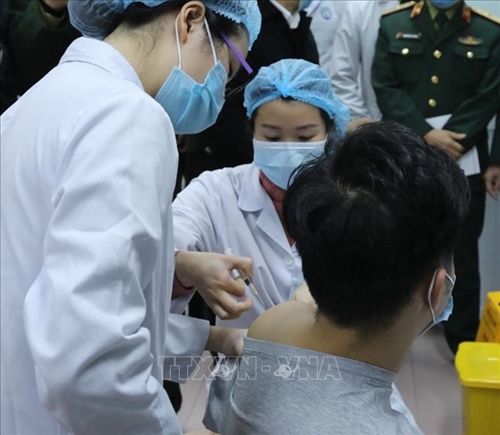
The first human trial of COVID-19 vaccine Nanocovax begins in Vietnam on December 17. |
The first candidates are among 60 volunteers, aged 18-50, selected for the first phase of the vaccine trials. They are divided into three groups for receiving three doses of 25 mcg, 50 mcg and 75 mcg.
With safety staying first in vaccine clinical trials, the first volunteers received the minimum dose of 25 mcg. They will stay at separate rooms at the Military Medical University for the first 72 hours for medical assessment. They will continue to be closely monitored at their residences for the next 56 days.
A team of more than 40 medical experts has been set up to deal with unwanted side effects after the vaccination.
“In the world, there have been very few vaccine trials with unexpected reactions, and we hope Nanocovax vaccine is no exception,” said Prof., Dr. Do Quyet, Director of the Military Medical University.
“To ensure the highest safety for the volunteers, we had to best prepare, even for the life-threatening circumstances,” he said.
“We pledge to assess [the vaccine] objectively, honestly, and transparently, and submit the proposals to the health ministry for later-stage trials,” he continued.
Source: VNA