August 22, 2021 | 17:07 (GMT+7)
Ministry of Health allows distribution of 30,000 Remdesivir vials
The Ministry of Health on August 21 allowed the distribution of 30,000 more vials of the antiviral drug Remdesivir to treat COVID-19 patients in a number of localities.
Recipients include 11 hospitals, 12 departments of health in cities and provinces consisting of HCM City, Binh Duong, Dong Nai, Tra Vinh, Vinh Long, Da Nang, Phu Yen, Khanh Hoa, Soc Trang, Ben Tre, Kien Giang and Hau Giang, and the health division of the Military Medical Department.
    |
 |
|
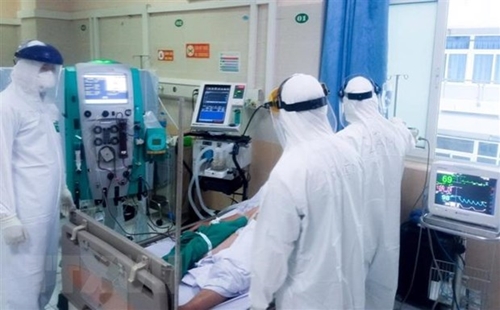
Treating a COVID-19 patient |
Earlier, 10,000 vials were distributed on August 8 for Ho Chi Minh City and a number of southern provinces. Another 30,000 vials of the drug were recently distributed on August 17 for 17 hospitals and health departments in the south.
Remdesivir was the first antiviral drug approved by the U.S. Food and Drug Administration (FDA) in October, 2020 for treating COVID-19 patients. It has the ability to shorten treatment times and speed up recovery for critically ill patients.
The drug has been approved and authorized for use in approximately 50 countries worldwide. It is now one of the world's leading specialized drugs.
On August 2, with the ministry’s guidance, conglomerate Vingroup had successfully negotiated for 500,000 vials of Remdesivir from Indian pharmaceutical company Cipla under licensing agreement from U.S.-based Gilead Sciences. The first batch arrived at Tan Son Nhat International Airport in HCM City on August 5.
Source: VNA