March 23, 2021 | 18:28 (GMT+7)
Fifteen more volunteers injected with COVIVAC candidate vaccine
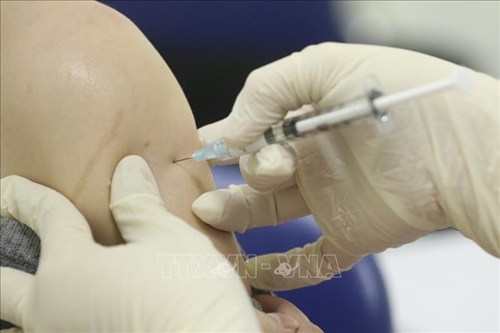
Fifteen more volunteers have been inoculated with Vietnam’s second homegrown candidate vaccine COVIVAC on March 23 as part of its ongoing human trials.
The candidate vaccine will be also injected into 15 other volunteers on March 25.
Earlier on March 15, the first six out of the total 120 volunteers took their shots of the vaccine, with their overall health checked on March 22. They are now in stable health conditions and doing their work as normal.
    |
 |
|
Volunteers take their shots of the vaccine. |
All the volunteers will receive their second shots 28 days after the first.
Developed by the Institute of Vaccines and Medical Biologicals (IVAC) since last May, COVIVAC is the result of cooperation between IVAC and a number of partners, including the Hanoi Medical University, the Icahn School of Medicine at Mount Sinai in New York City, the University of Texas in Austin, and the US-based health organization PATH.
Using primary chicken embryo cell culture - a technique the institute used previously to successfully produce seasonal flu vaccines - it is the second “made-in-Vietnam” candidate vaccine tested on humans.
The Nanogen Pharmaceutical Biotechnology Company earlier completed the first phase of human trials of its NanoCovax vaccine and began the second phase on February 26.
Source: VNA